They haven't found any liquid neon on Pluto yet (one of the other liquids suggested as a possible habitat for life by William Bain). Jeff Kargel's idea of neon rivers on Pluto may seem a rather exotic suggestion, but though neon is very rare on Earth, it is actually similar in cosmic abundance to nitrogen, behind hydrogen, helium, oxygen and carbon, and also similar in solar abundance too (neon's solar abundance is not very well determined with many papers revisiting this question).
It's a light gas, lighter than air, and very volatile which is why there isn't much left in the inner solar system (it is a heavier atom than nitrogen, atomic mass 14, but nitrogen comes as a molecule N2, so that's 28 atomic mass units compared to Neon's 20). Pluto is a little warm for neon to be liquid. REX estimated a temperature next to the surface of 45±3 K on occultation exit, and 37±3 K on occultation entry. The lower temperature was measued above Sputnik Planum, an area of nitrogen ice, and is close to the saturation temperature of nitrogen. So that suggests a cold layer hugging the nitrogen ice. At any rate, it's far too warm for liquid neon right now.
So, Pluto is rather warm for neon rivers and seas, but it might easily be abundant in cooler locations further out in the Kuiper belt. The triple point of neon is at 24.5 K and 0.4 bars.
Stacy McGaugh wrote an intriguing speculative piece about the possibility that Sedna could have a neon atmosphere when close to the Sun,
He writes:
"The following is a speculative idea about the possibility of a thin, hazy neon atmosphere on the remote planetoid Sedna. It is almost certainly wrong. I post it as an intriguing (if remote) possibility, and to stimulate thought more generally on the possible role of the cosmically abundant element neon in frigid places like the outer solar system (well beyond the Kuiper belt) and dusty star formation cocoons."
He talks about how it's possible it could have a neon atmosphere when it is closest to the sun, and it could have crossed the solid / gas boundary for neon when it got closer than 120 au and that if the atmosphere gets thick enough it could even have liquid neon. It's not so easy for neon to become part of a forming object though, because it is so volatile, and it doesn't form bonds with anything else, being a noble gas. So as the condensing gas warms up the neon would evaporate. However we do have some neon on Earth, and it's also found in meteorites and in comets. He concludes that these "all combine to suggest that it is worthwhile to consider the possibility of a neon atmosphere on Sedna even if it may seem farfetched"
So, though they didn't find neon rivers on Pluto - I wonder if we will find any KBO's with a neon atmosphere? If so, what about a world in the outer solar system with a neon atmosphere, and liquid neon lakes and rivers and a neon precipitation cycle? If so that may be another place to look for William Bains hypothesis of low temperature silicon based biochemistry mentioned in Life in liquid nitrogen (above)
Life in liquid hydrogen or liquid helium
Another of my speculative sections - MID EDIT - see end of the section.
Eventually as you get further out in the Kuiper belt and into the Oort cloud then the temperatures are so low only hydrogen and helium ae available as liquids. William Baine was interested in orbjects of 1,000 km diameter or larger with a potential for an atmosphere.
As we saw above, the idea is to use chemical reactions that continue too rapidly to be useful for life at our temperatures. William Baine suggests silanols. The silicon - hydrogen bond is destroyed within hours at mammalian temperatures in presence of water, not nearly as stable as the carbon - hydrogen bond, but it's just what you want at such low temperatures and in non polar solvents. As he wrote in one of his papers:
"... Oceans of cold, non-protonating fluids, and specifically liquid nitrogen, would be more favorable for silicon life. In such environments, the inherently greater reactivity of silicon-based chemicals could be an advantage, enabling “living” processes to occur at greater than geological speeds at the relatively lower temperatures where such fluids are stable."
He works out that in those conditions, with the background radiation of the Big Bang at 2.7 °K and Helium boiling at 4.2 °K that (page 147 of this paper).
"there's no reasonable set of circumstances where a plaent's surface is likely to be cold enough to maintain a temperature of < 4.2 °K and where the planet is massive enough to retain a significant atmosphere."
He doesn't discuss the possibility of liquid helium on smaller cooler bodies with no internal heat. So is that possible? I can't find much about this idea, but the science fiction writer Stephen Baxter populates a fictional airless distant tiny Kuiper belt object just a hundred miles across (160 km) with helium condensing on its surface, and with creatures with helium instead of blood in his short story "Sun people".
William Baines also mentions the idea of liquid hydrogen at the beginning of the paper, and doesn't rule it out, but doesn't go into it any further. With his biochemistry based on silanols, it could support life even when it is not supercrtiical.
Liquid hydrogen is rather easier to form, with a freezing point of 14 °K, and a boiling point at Earth's atmospheric pressure of 20°K (Hydrogen properties). Looking at other pressures, then liquid hydrogen can only exist at temperatures between its triple point and its critical point of 33 °K (I'm talking here about normal rather than supercritical liquid hydrogen). That's not far below the 37±3 °K measured on Pluto above the nitrogen ice of Sputnik Planum.
Techy details: those figures depend on the deuterium content. These two temperatures can be increased by up to 5 °K for pure deuterium.
The triple point of hydrogen - the point where the gas, liquid and solid phases all meet - is fixed by definition at -259.3467 C = 13.8033 K according to the International Temperature Scale of 1990.
The pressure for that triple point is 0.0704 atmospheres
These figures depend on the deuterium content. In that definition, the deuterium content is assumed to be 0.15 micromoles per mole of hydrogen typical of hydrogen on Earth (in experiments on Earth it actually can vary by microkelvins depending on the exact deuterium content of the hydrogen)
The triple point of deuterium is 18.7 K at atmospheric pressure and its critical point is 38.34 K.
It also depends on the spin states - hydrogen occurs in two spin isomers depending on the spin states of the protons, whether aligned (ortho) or anti aligned (para). The temperatures can vary for the two isomers, depressed by about 0.1 K for the para hydrogen compared to normal (mixed spin state) hydrogen.
Those are temperatures that would be normal in the remoter reaches of the Kuiper belt and in the Oort cloud. Also if they are cold enough they can retain an atmosphere of hydrogen.

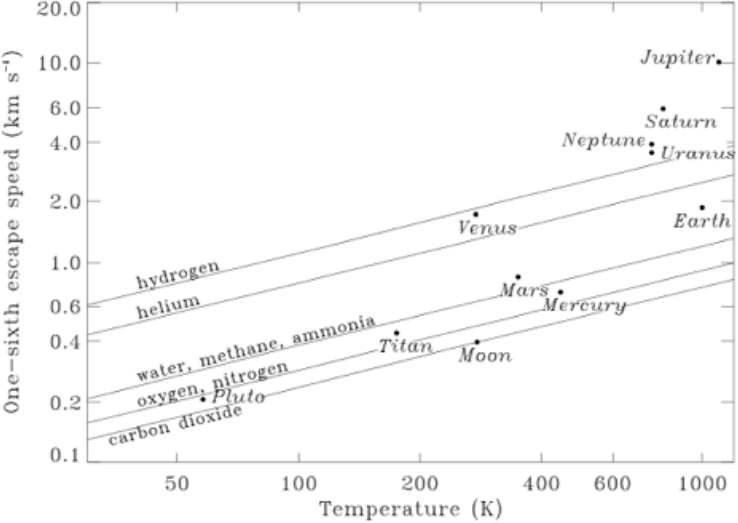
Figure 3 of this paper. This shows the gases that are prone to escape from the atmosphere of worlds of various sizes through "Jeans' escape" - that is to say just through the loss of individual molecules whose velocity due to temperature is enough to reach escape velocity. The lines are based on the 'rule of thumb' that Jeans escape velocity is important if the velocity at the "exobase" is more than a sixth of its escape velocity. There the exobase refers to the height in the atmosphere where collisions between the molecles become rare. For Earth, this is at 500 - 600 km.
From this diagram, any larger planets in the Oort cloud or outer Kuiper belt surely could hold onto hydrogen if they lose it only by Jeans' escape. Gases can also escape via thermodynamic escape, a heating up of gas through absorption of UV from sunlight, leading to bulk flows of the gas into space starting from below the exobase, which makes it more complex. However Pluto is losing nitrogen at the Jeans' escape rate, not the thermodynamic escape rate, corresponding to a loss of about 6 cm of nitrogen ice over the age of the solar system. See the section on Escape towards the end of this paper.
So, it seems at least possible that worlds out there as small as Earth and smaller could hold onto a hydrogen atmosphere, and potentially, if the temperatures are right, have a hydrogen ocean.
So, could there be any objects out there with enough gravity to form surface oceans of liquid hydrogen?
Mike Brown (one of the discoverers of Sedna and Eris and numerous other objects in the outer solar system) in one of his talks predicts that we are likely to find planets beyond Neptune that are Mercury, Mars or even Earth sized. The video is here:
Paraphrase: "Sedna has a twelve thousand year orbit around the sun. And we happened to find this one almost at the closest point it ever gets to the sun. Not by coincidence, because there is only about a 200 year period, where we could have seen it."
"So, 200 years out of 12,000 years means a 1 in 60 chance of finding it. So either we are very lucky, or since we only had a 1 in 60 chance of finding it, probably there are 60 of them and we just found the one that happened to be close.
"Maybe it's not 60. Maybe it's 30 and we got a little bit lucky. Maybe there are 90 and we got a little bit unlucky. But there are a lot of objects in this very distant region where we never knew of anything before.
"Now the fun thing to think about is, if there are 60 of these, about three quarters the size of Pluto, like Sedna, there are probably
- 30 objects the size of Pluto.
- 10 objects that are twice the size of Pluto,
- Two or three objects that are three or four, and maybe even five times the size of Pluto in this region here.
"Our big goal now, and one of my current graduate student's PhD thesis is to find these objects. If there are some big objects, two or three or four times the size of Pluto, these things are the size of Mercury, these things are the size of Mars, these things are the size of the Earth.
"I am willing to go out on a limb and say that we will find something like that the size of Mars somewhere in this region of space."... I think if you find something the size of Mars, something the size of the Earth, I think most people are going to want to call it a planet.If we are really lucky we will find them in two or three years. They have to be a little bit close. Otherwise it is going to take another ten or fifteen and some bigger telescopes. But that's where we are headed and that's where this whole field is I think going next. "
We haven't discovered any of these yet, but here for instance is a paper from 2017, by Kathryn Volk and Renu Malhotra, building on earlier work by Lykawka&Mukai in 2008, suggesting the possibility of a Mars sized object at a distance of 65–80 au to explain the “Curiously warped mean plane of the Kuiper belt”. That’s based on the observation that the Kuiper belt bodies are in a plane offset by about eight degrees from the “Invariable plane” of our planets. For non technical account of their research by the astrophysicist Brian Koberlein, see Goodbye Planet Nine, Hello Planet Ten
There's also the possibilty that "Planet 9" consists of not just one but several objects: Worlds Beyond Pluto --"There's Not Just Planet 9, But Several Planets Beyond" and then you also have the possibility that while searching for it, they turn up smaller objects as happened for Pluto with Percival Lowell and Clyde Tombaugh's search.
So, though we haven't found them yet, such objects are certainly possible and it's maybe even rather likely that we find them, probably in the next decade or two. If these objects exist they could be the most common type of rounded object in our solar system. Especially if there are more of those objects even further out in the distant Kuiper belt and Oort cloud. I happen to think that we should follow the geophysical definition and call them all planets, including Pluto, Eris, Ceres, Haumea our Moon and so on. See my article on the subject here. However, one way to be neutral about that debate is to just call them "worlds". If you include the smaller worlds, then the small worlds in our solar system already outnumber the better known planets.
So now, if any of those worlds, the larger ones, have surface oceans, not of water, but of liquid hydrogen, then they could even be examples of the most common type of world in our galaxy to have surface oceans. In our solar system then the only examples would be Earth itself, Titan, and then any distant worlds with neon or hydrogen oceans. So is such a world with a liquid hydrogen ocean possible?
I've found one mention of the idea so far, in a 1980 book "Life Beyond Earth" by Gerald Feinberg. I haven't read it yet but have ordered it. I will update this once I find out more.
Also - I've put this up as a question on space.stackexchange.com, but so far had no replies.
Could there be life at such very low temperatures as liquid hydrogen? Well it's stil la fair way above absolute zero. I'm just reporting what William Bains says here. He suggests that though life would probably get off to a slower start in such conditions, in the early stages of evolution, that once it has evolved it should be no harder to imagine than at our temperatures. He says that (I leave out his inline cites for easy reading):
"Can chemistry happen fast enough in such cold environments for life to start, and to be maintained? Reaction rates for the initiation of life will be limiting—simple reactions such as polymerization of HCN, believed to be important in some scenarios of terrestrial biogenesis, happen on time scales of the same order of magnitude as the age of the Solar System at Titan’s surface temperature. However, catalysis— even very weak general acid/base catalysis—can speed this up dramatically. If such chemistry was possible, then selection of chemistry that was selfcatalyzing would ensure the development of more efficient catalysts. This is the route by which peptides and proteins are created in Russell’s scenario. "
"Terrestrial enzymes can catalyze reactions extremely efficiently, such that some apparently abolish the activation energy entirely so that the enzyme-catalyzed reaction rate approaches the diffusion-limited maximum possible rate, as do other processes such as protein folding in some circumstances as well as the more obviously diffusional protein:protein binding interactions. Diffusion-limited chemistry is not unfeasibly slow in liquid ethane, nitrogen, or hydrogen, because values of the diffusion-limited reaction rate constant kx are linearly proportional to temperature (T) and inversely proportional to viscosity."
"Terrestrial enzymes have been shown to be catalytically active down to temperatures of -100°C (170 °K), using a variety of mixed polar solvents; below 170 °K these solvents become so viscous that diffusion limits effectively stop chemistry from happening, a limit that would not apply to the liquids in Table 5 [table of liquids such as neon, hydrogen etc]."
"Thus the origin of life in such circumstances would be slower and more dependent on the presence of endogenous catalysts. However, maintenance of life after that would be no harder to imagine than maintenance on Earth. It is notable that the “rate of living” of terrestrial organisms is not usually dependent on ambient temperature; the natural rate of growth of bacteria in the Antarctic seas and in black smoker volcanic vents is determined by competition for nutrients, not on how fast their chemistry can run. "
(from page 151 of his "Many Chemistries Could Be Used to Build Living Systems")
We also saw the possibility of Supercritical liquid hydrogen layers in gas giant atmospheres (above), which first becomes possible above 12.8 atmospheres and 33.3 °K. So could we get supercritical liquid hydrogen in these oceans as well? This could only happen rather deep in a surface ocean of liquid hydrogen as with a density of only 70 kg / cubic meter it is 14 times less dense than water. With a mass of 10.8 meters depth of water equivalent to one atmosphere in pressure, it would take 10.8*(1000/70.8)*12.8/1000 = 1.95 km depth of a liquid hydrogen ocean to have a supercritical layer at the bottom. On the other hand, liquid hydrogen trapped under low density amorphous ice with density 0.94 that of water would be supercritical at 10.8*(1/0.94)*12.8 = 147 meters.